Journal of Hepatology
Volume 58, Issue 4 , Pages 643-645, April 2013
Maria-Carlota Londoño, Sabela Lens, Xavier Forns
Liver Unit, Hospital Clínic, IDIBAPS, University of Barcelona, Barcelona, Spain
Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBEREHD), Barcelona, Spain
Received 11 December 2012; received in revised form 7 January 2013; accepted 8 January 2013. published online 16 January 2013.
See Articles, pages 646–654 and pages 655–662
Developments in the treatment of chronic hepatitis C over the last 2years have been remarkable. For the first time ever, we are now certain that this chronic infection can be cured without the need of interferon and ribavirin. Gane and colleagues provided the proof of concept that oral antiviral therapy with two direct-acting antivirals (DAAs) without interferon can suppress viral replication [1]. In their study, they showed that the combination of an NS5B nucleoside polymerase inhibitor (RG7128) and an NS3 protease inhibitor (danoprevir) had potent antiviral activity even in null responders; some patients achieved undetectable HCV-RNA only 14days after treatment initiation. Unfortunately, the combination of DAAs in this study was limited to 2weeks and was followed immediately by treatment with peginterferon and ribavirin, thus preventing the assessment of sustained virological response to an interferon-free regimen [1]. The combination of the protease inhibitor asunaprevir with the NS5A inhibitor daclatasvir is the first oral interferon-free regimen proved to be effective [2], [3]. In the study by Lok et al. [3], 11 previous null responders received both drugs for 24weeks and a total of 4 patients (2 of 9 with HCV genotype 1a and 2 of 2 with genotype 1b) achieved a sustained virologic response (SVR). In the study by Chayama et al. [2], 11 genotype 1b null responders underwent the same interferon-free regimen and the 9 individuals who completed 24weeks of therapy achieved SVR.
In this issue of the Journal of Hepatology, Suzuki et al. [4] evaluated the efficacy of dual therapy with asunaprevir and daclatasvir in 43 subjects infected with genotype 1b considered poor candidates for current treatment for hepatitis C (21 null responders and 22 ineligible or intolerant to interferon-based therapy). SVR at 12 and 24weeks was 90% for null responders and 64% for ineligible/intolerant to interferon-based therapies. Treatment was well tolerated and virological failures were only observed in the cohort of ineligible/intolerant patients (3 breakthroughs and 4 relapses). In the accompanying manuscript, Karino et al. [5] characterized the escape viral mutations in patients experiencing virological failures. The authors found that NS3 and NS5 resistance-associated variants (RAVs) were detected together at the time of virological failures.
One of the strengths of the study of Suzuki et al. [4] is that it deals with difficult-to-treat patients: well-documented null responders and patients who are intolerant or ineligible to interferon. Although the latter group was rather heterogeneous (individuals older than 70years, with depression or other co-morbidities), this profile of patients represents a significant proportion of our current candidates to antiviral therapy. Obviously, the combination of peginterferon, ribavirin and a first generation protease inhibitor (boceprevir or telaprevir) is not an option for patients with absolute contraindications to interferon and it is also a poor choice for individuals with co-morbidities or those who are old. In Japan and China, hepatitis C virus expanded decades before that of the United States and Europe [6]. Therefore, candidates to antiviral therapy in Asia are often older than corresponding patients in Western countries. Older age is not an absolute contraindication for an interferon-based therapy. A French group showed good efficacy in a small group of patients older than 65years treated with pegylated interferon and rivabirin [7]. Nevertheless, other studies have demonstrated a trend towards lower SVR rates, as well as higher rates of dose reductions and discontinuations of therapy in this population as compared to younger individuals [6], [8]. Currently, there are no data on the safety and efficacy of triple therapy in old patients. In the CUPIC French cohort, cirrhotic patients up to 83years old have been included: though the number of severe adverse events using triple therapy seems clearly higher than those reported with peginterferon and ribavirin alone [9], a specific analysis in older patients has not been performed.
Similarly, triple therapy is not an ideal alternative for most previous null responders, since SVR rates in this subpopulation rank only between 30% and 40% [10], [11]. Moreover, subgroup analyses from the REALIZE study [10] suggest that in cirrhotic null responders SVR is below 15%. In order to accomplish the definition of “difficult-to-treat” patients, it would have been interesting if the study by Suzuki et al. had included patients with advanced liver disease (biopsy-proven cirrhosis was an exclusion criterion).
Response rates obtained in this study using daclatasvir and asunaprevir can be considered excellent. It is surprising though, that the only virological failures reported were in the group of intolerant/ineligible patients [5]. Although the small number of patients precludes any definitive interpretation, there are several potential explanations. Firstly, it is important to notice that 10 out of the 21 null responders (sentinel cohort) received a significantly higher dose of asunaprevir, which was not the case in any of the 22 intolerant/ineligible individuals. Second, patients experiencing virological failure had below-median daclatasvir and asunarpevir levels, but this was also the case for other individuals who achieved sustained viral clearance. A lack of compliance did not seem to play a major role in the lower efficacy in this group (though cannot be completely excluded). A more interesting hypothesis is the potential effect of pre-existing resistance-associated variants (RAVs). In a complementary manuscript, Karino et al. [5] performed a careful characterization of virological escape mutants in patients included in the first study. Interestingly, most patients experiencing viral breakthrough or relapse had daclatasvir RAVs at baseline, being NS5A-Y93H the predominant polymorphism in all 3 patients with virological breakthrough and in 2 of the 4 relapsers. The global prevalence of this variant is around 4% [5], [12] and may be higher in genotype 1b-infected patients (∼10%). Indeed, NS5A-Y93H was found at baseline in five other patients who achieved SVR in this study.
In every patient with virological failure, resistant variants to both agents emerged together at the time of failure (NS3-D168A/V and NS5A-L31M/V-Y93H). At baseline, a combination of these NS3 and NS5A variants was not detected by clonal sequencing; however, their presence at low levels cannot be excluded due to the limited number of clones analyzed. Currently, assessment of minor NS3 plus NS5A RAVs from the same RNA sequence is not possible by ultra-deep sequencing technologies, since the size of the analyzed fragments is still a limitation (a fragment of ∼4000 base pairs encompassing NS3, NS4 and NS5A is far too large for the current technology).
A final point analyzed in the accompanying manuscript by Karino et al. was the persistence of RAVs after treatment interruption [5]. This is a very relevant topic, since it may impact future treatment options in patients who develop drug resistance. As reported with other protease inhibitors, asunaprevir-resistant NS3-D168 substitutions generally decayed during the follow-up period, which implies a lack of replicative fitness compared to the wild type virus in the absence of selective pressure (drug). This was also reproduced in the replicon system, where double NS3 RAVs (D168V plus Q80L or S122G) had a replicative ability similar to the D168V variant alone. Obviously, a more thorough sequence analysis using ultra-deep pyrosequencing would be necessary to fully establish the dynamic decay of these RAVs, after treatment interruption and to make sure that these variants do not remain enriched for longer periods relative to baseline. In fact, a small study including 5 patients who were first treated with simeprevir monotherapy (5days), and then retreated more than 1year later with pegylated interferon, ribavirin and simepervir, analyzed the potential clinical implications of the presence of RAVs. In this study [13], 3 patients achieved SVR and 2 did not. Deep sequencing indicated low-level persistence of simeprevir RAVs in the 2 patients who did not achieve SVR. We do not know if the presence of these resistant strains at low levels explained the lack of response to re-treatment. What is really interesting in the study by Karino et al. [5] is that in some individuals, NS5A variants associated with daclatavir resistance persisted for at least 48weeks after treatment interruption. As already mentioned, longer follow-up studies are important to establish the clinical impact of these more fitted resistant strains in case these patients will be retreated with NS5A inhibitors.
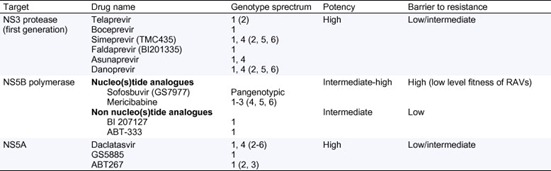
Overall, the ideal combination of DAAs is still unknown, but some of the inherent characteristics of the antiviral agents may help predict which combination will be more effective (Table 1). The inclusion of a nucleo(s)tide NS5B polymerase inhibitor in a combination seems reasonable [14]. These drugs offer a high barrier to resistance (RAVs have a very poor fitness), are pangenotypic and have proved to be very effective in several phase 2 trials. The simple combination of sofosbuvir and ribavirin for 12weeks appears to be extremely successful in naïve genotype 1, 2 or 3 patients (though this combination using such a short regimen is insufficient to cure previous null responders) [15]. Combinations including more than 2 DAAs targeting different viral proteins also seem a good approach. Recently, a study including both naïve and null responder genotype 1 patients assessed the efficacy of ABT450/r (ritonavir-boosted NS3 inhibitor), ABT267 (NS5A inhibitor), ABT 333 (NS5B non nucleoside inhibitor) and ribavirin. This combination achieved SVR12 rates close to 100% in naives and around 90% in null responders [16]. Unfortunately, patients with advanced liver disease have not yet been included in these studies. The only data regarding cirrhotic patients treated with oral regimens comes from the SOUND-C2 study, where an NS3 protease inhibitor (faldaprevir), a non-nucleoside NS5B inhibitor (BI207127) and ribavirin were combined in genotype 1 naïve patients: reported SVR12 rates in cirrhotics were around 60% [17].
Table 1. Characteristics of direct antiviral agents approved for hepatitis C treatment or entering phase 3 studies.
Genotypes in parenthesis indicate documented activity in vitro.
Within the next few years, we will certainly witness more progress. When choosing a combination of antiviral agents, we will need to take into consideration a certain number of variables: potency, genetic barrier to resistance, range of activity (pangenotypic or not), potential drug–drug interactions. Importantly, safety and simplicity of the regimen will also be very relevant. Up to now, most of the oral compounds appear to be safe and well tolerated by most patients, but until large phase 3 studies are finished, safety needs to be closely monitored. Most of our current knowledge on interferon-free regimes is based on phase 2 trials including small numbers of patients. Added to which, we still have very little information on the safety and efficacy of these regimens in difficult-to-treat subjects, particularly in null responders with advanced fibrosis or cirrhosis, or in special populations such as transplant patients with hepatitis C recurrence. Over the next 2–3years, we will start to see data on large cohorts (phase 3 studies) and in small series of really difficult-to-treat individuals and in special populations. By then, it will be easier to answer the question: “are we there?”.
No comments:
Post a Comment